The past several decades have seen a gradual but definitive shift in our view of the sinonasal epithelium from a passive barrier to an active immunologic organ with both innate and adaptive components (Figure 1). In parallel, multiple investigations have demonstrated that the inflammatory profiles associated with CRS are often predicated upon the dysfunctional regulation of these complex mechanisms with both downstream and reciprocal sequelae.
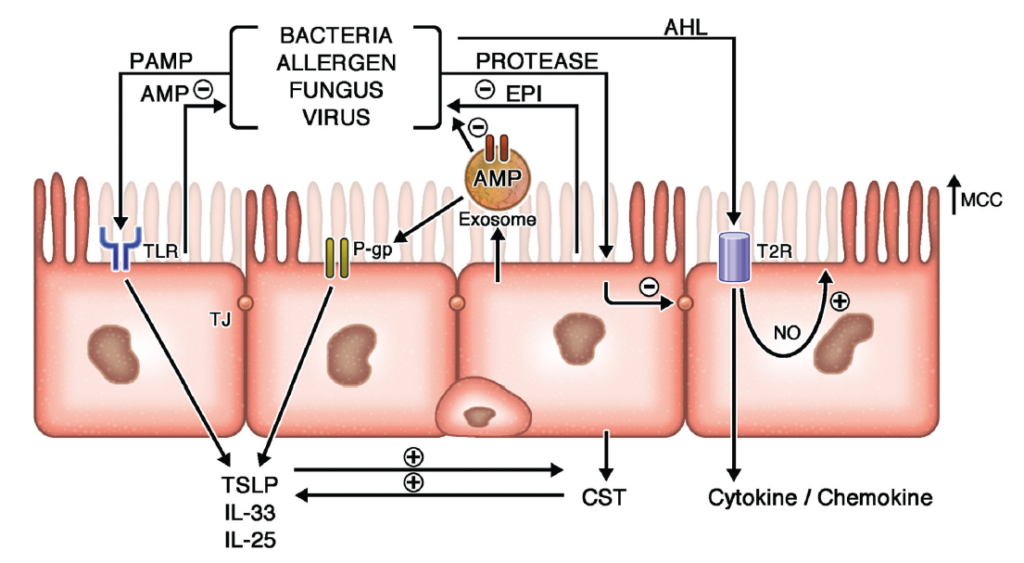
Figure 1. Epithelial Contributions to CRS
Extrinsic pathogens and irritants interact with multiple epithelial receptors to induce both innate and adaptive immune responses. Protease exposure induces tight junction (TJ) dysfunction and secretion of endogenous protease inhibitors (EPIs) which neutralize extrinsic proteases and stimulate type 2 inflammation. Similarly, pathogen-associated molecular patterns (PAMPs) interact with Toll-like receptors (TLRs) leading to apical anti-microbial peptide (AMP) secretion as well as epithelial derived cytokine secretion. P-glycoprotein (P-gp) functions to clear the cytoplasm of environmental toxins while reinforcing epithelial cytokine release. Epithelial derived exosomes support these innate immune responses by shuttling AMPs directly to mucus borne pathogens while promoting the inter-epithelial transfer of P-gp. Bacterial derived quorum sensing molecules, such as acyl-homoserine lactone (AHL) compounds, also interact with bitter taste receptors such as T2R38 to induce bacteriocidal nitric oxide (NO) release, enhanced mucociliary clearance (MCC), and cytokine/chemokine secretion. (Kato A, Schleimer RP, Bleier BS. Mechanisms and pathogenesis of chronic rhinosinusitis. J Allergy Clin Immunol. 2022 May;149(5):1491-1503. doi: 10.1016/j.jaci.2022.02.016. Epub 2022 Mar 1. PMID: 35245537; PMCID: PMC9081253.)
Protease Inhibitors in Chronic Rhinosinusitis

Cystatin 1 and 2 (CST1 and CST2) are profoundly and focally overexpressed within nasal polyp epithelium, secreted in exosomes, and correlated with CRSwNP disease severity. Chronic recombinant CST1 exposure is capable of inducing a type 2 inflammatory response in healthy murine mucosa in a time-dependent manner along with concomitant P-gp overexpression. Knockdown of the immunomodulator P-gp at the level of the epithelium results in


Nocera AL, Mueller SK, Workman AD, Wu D, McDonnell K, Sadow PM, Amiji MM, Bleier BS. Cystatin SN is a potent upstream initiator of epithelial-derived type 2 inflammation in chronic rhinosinusitis. J Allergy Clin Immunol. 2022 Oct;150(4):872-881. doi: 10.1016/j.jaci.2022.04.034. Epub 2022 May 31. PMID: 35660375; PMCID: PMC9547833.
The role of P-glycoprotein in Chronic Rhinosinusitis
Permeability glycoprotein (P-gp) is an active efflux membrane transporter that has been researched extensively due to its ability to confer multidrug resistance in a wide range of cancers. The gene for P-gp, often referred to as ABCB1 or MDR1, is located on chromosome 7q21.12. P-gp has an impressively broad substrate specificity and is thought to be responsible for extruding xenobiotics and cellular metabolites, as well as maintaining tissue barriers at the blood-brain interface and gastrointestinal epithelium. P-gp is also thought be involved in regulating immune responses and is able to influence the secretion of cytokines and chemokines.
In our lab, we first discovered the presence of localized P-gp overexpression within nasal polyps in 2012. Since then, we’ve shown that this overexpression leads directly to the hypersecretion of type 2 helper T-cell pro-inflammatory cytokines which, in turn, perpetuate the inflammatory cascade. Additionally, this overexpression leads to resistance to corticosteroid treatment in these patients.
More recently, we have demonstrated that epithelial derived exosomes are capable of shuttling functional P-gp between cells, which may contribute to the field inflammatory effect characteristic of the disease.
Feldman RE, Lam AC, Sadow PM, Bleier BS. P-glycoprotein is a marker of tissue eosinophilia and radiographic inflammation in chronic rhinosinusitis without nasal polyps. Int Forum Allergy Rhinol. 2013 Aug;3(8):684–7.
P-gp participates in the non-canonical regulation of cytokine secretion within nasal polyps and may represent a druggable target. Verapamil is a calcium channel blocker, which binds to the alpha subunit of L-type voltage dependent calcium (Cav1) channels and blocks the influx of calcium ions into the host cell. In addition to this function, Verapamil was also one of the first inhibitors of P-gp to be identified. Several studies, including studies by our group, have reported that Verapamil is capable of modulating inflammatory responses in human T cells, animal models of asthma, and nasal polyps. Using an organotypic explant model, we have shown that Verapamil has similar effects to dexamethasone in its ability abrogate both IL-5 and IL-6 secretion. While Verapamil is a cardioactive drug, it is considered the first-line prophylactic drug for cluster headache and is usually well tolerated by otherwise healthy patients.
Our lab completed a randomized, double-blind, placebo-controlled pilot study of Verapamil (80mg TID) in patients with CRSwNP (n=10 per group) following a four week washout of local and systemic corticosteroids. The primary endpoint was SNOT-22 confirmed with 10cm Visual Analog Score(VAS) score reduction at eight weeks. Secondary endpoints included difference in Lund-Mackay computed tomography and change in Lund-Kennedy endoscopy scores (LMS and LKS). This was the first study to explore P-gp modulation as a treatment for CRSwNP. The effect size of Verapamil with respect to improvement in SNOT-22 was comparable to previously reported values using both biologic agents and corticosteroids. While the small population size was not adequately powered to confirm efficacy, the significant improvement evident across multiple outcome measures opens the door to further exploration of the P-gp inhibitory strategy. Our data also demonstrated that low dose Verapamil monotherapy is well tolerated by patients with less side effects and lower costs than those reported in previous studies using biologic agents.

